In India almost 4 to 5 % women are Rh negative. Importance and knowledge of Rh immunoprophylaxis, Rh Alloimmunization and Hemolytic disease of new born is lacking and some time neglected and over sighted possibly due to economical reasons. Worldwide, with the introduction of routine prophylaxis, the incidence of RhD Alloimmunization has reduced dramatically. However, in India a lot still needs to be done. There is gross underutilization of Anti D prophylaxis in India. Deka D et al observed that failure to administer post natal anti D prophylaxis was responsible for RhD Alloimmunization in more than 50% of cases, followed by failure to administer Anti D after MTP (10%) in India. If fetal anaemia is diagnosed early before hydropic changes sets in fetal survival is up to 80-90 % after intravascular fetal transfusion of RBCs but if hydropic changes are set in , survival reduces to 25 -35 % only.
Following methods are used today for assessing the mother and the fetus for knowing the extent of the disease and its sequel:
Antenatal management of the Rh-negative pregnant woman begins with screening.
First step in the screening is to know the Blood Group & Rh status of Father with genotyping if possible. If the father is Rh negative, Alloimmunization in fetus is not possible, so no further specific investigation required as far as Rh Alloimmunization is concern. If father has Rh positive status, he could be homozygous where fetus is 100 % Rh positive or Heterozygous where fetus will have 50 – 50% chance of Rh positive/ negative status as the fetus will inherit one copy of the RHD gene and therefore Rh Alloimmunization may occur during pregnancy. In India it is not a routine practice to do invasive procedure to determine the fetal blood group from fetal DNA but if warranted amniocentesis should be preferred than Chorionic villus biopsy as the later procedure has more chance of FMH and so it will worsen the disease that to early in the pregnancy. (Moise KJ Jr, Carpenter RJ Jr. Chorionic villus sampling for Rh typing. Am J Obstet Gynecol 1993; 168:1002–3)
In future fetal cells/ DNA from maternal blood could be of help in detecting the Fetal Rh status without doing the invasive procedure.
Second step is – all patients should undergo an Indirect Coombs Test (ICT) at the earliest after diagnosing Rh negative group and should be repeated at 26 and 32 weeks of gestation. ICT allows detection of detection of RhD and other atypical antibodies that can cause hydrops. Counseling regarding the Rh negative group and its implications in her obstetric carrier to the women is must. If the antibody screening does not show evidence of Alloimmunization, the patient should receive anti-D immune globulin prophylaxis at 28 weeks of gestation as describe later. If mother remains non-immunized she must receive the post partum prophylaxis if needed.
Testing every 6 weeks will avoid missing the development of antibodies in the occasional patient who becomes immunized before delivery and will prevent the rare poor fetal outcome that may result from inadequate surveillance.
Ultrasound is the master key in the management of all immunized pregnancies, useful in following ways.
Early and first trimester USG will be of great help in determining exact gestational age of the fetus as complete management will be dependent on the gestational age of fetus.
Ultrasound evaluation of following should be done in cases of Rh Alloimmunization.
Accumulation of 30 to 50 ml fluid in peritoneal cavity will make the visualization of both sides of the bowel wall and lucent outlining of organs such as the bladder and stomach. Accumulation of 100 ml in the abdominal cavity will make ascites marked.
Increase in the abdominal circumference could be because of Ascites, Hepatospleno-megaly (reflecting increased extra-medullary erythropoiesis).
Placental thickening occurs. > 5 cms thickness is associated with significant anemia
Cardiomegaly also may be visible given the demands on the myocardium necessary to maintain perfusion.
Increase in amniotic fluid index can provide important clues to early fetal anemia which occurs due to increased COP as a compensatory mechanism. It may be followed by polyhydramnios. Further, deterioration is later manifested by oligohydramnios, an ominous finding.
The course of deteriorating erythroblastosis Fetalis is marked by polyhydramnios, followed by placentomegaly, hepatomegaly, ascites, and finally, generalized fetal hydrops
Prediction of fetal anemia is the key of improving the outcome of the fetus.
Although FBS will give the most accurate determination of fetal hematocrit, it is not practical to do such invasive procedures due to its complications. So researchers started searching for the parameter which should be non-invasive, accurate and most reproducible.
Chitkara and coworkers reveled that only frank hydrops was predictive of severe fetal anemia (hematocrit less than 15%).
Nicolaides and associates reported poor predictive power of placental thickness and amniotic fluid volume.
Splenomegaly, Cardiomegaly Splenic artery Doppler were also tried but with poor sensitivity was found.
Iskros and coworkers showed the ray of hope by using the Doppler flow analysis of the umbilical veins to show that elevation in maximum velocity of umbilical vein flow preceded hepato-splenomegaly.
Iskros applied two principals. First – Poiseuille’s law and second-hemodynamic changes in the fetal circulation that would be associated with mild to moderate anemia. It was assumed that fetal blood vessel cross-sectional area remains unchanged during the Rh alloimmunization.
As anemia progresses, fetal COP increase up to 45%, and but the heart rate remains unchanged which leads to increase in stroke volume. On other hand reduction in fetal Hb will leads to reduction in the viscosity. When both these effect combine together will increase the flow in the circulation in the vessel (flow being the product of velocity and cross-sectional area).
The Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses reported the use of peak velocity measurements of the middle cerebral artery (MCA) in predicting moderate to severe anemia.
Early and first trimester USG will be of great help in determining exact gestational age of the fetus as complete management will be dependent on the gestational age of fetus.
Ultrasound evaluation of following should be done in cases of Rh Alloimmunization.
Authors concluded that measurement of peak velocity in fetal MCAs was an accurate and noninvasive tool for identifying the fetus at risk, thereby improving the identification of those requiring invasive procedures such as transfusions.
In multicentre study of the above findings authors reported that MCA PSV predicted moderate to severe anemia with a sensitivity of 88% and specificity of 87% (NPV-98%; PPV-53%).
The fetal middle cerebral artery closest to the maternal skin should be evaluated using a minimal angle of insonation; the Doppler gate is placed over the vessel just as it bifurcates from the carotid siphon.
Mari et al has done tremendous work in fetal anemia and its management. Mari et al developed a formula for predicting the fetal hemoglobin using the middle cerebral artery peak velocity (2002) by following formula.
The correlation between the fetal Hb and MCA PSV becomes more accurate as the severity of anemia increases.
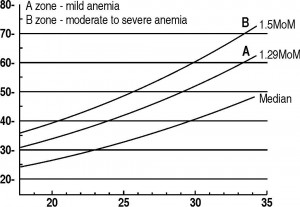
Haemoglobin multiples of the median (MoM)= 0.6835 + MCA-PSV MoM x 1.2794 – 1.2885MCA-PSV2 + 0.2861 x MCA-PSV3.
MCA PSV measurement had no discriminative power after 35 weeks’ gestation and after 2 or more fetal transfusion already done.
after an intra-uterine transfusion (IUT) the characteristics of fetal blood are altered because adult red cells are smaller and less rigid and they display an increased tendency for erythrocyte aggregation. Therefore it became pertinent to examine the usefulness of MCA PSV following IUT. But applying a change in the established cutoff level for MCA PSV from 1.50MoM (multiples of the median) in the fetus never transfused to 1.69MoM in previously transfused fetus may be of help.
MCA PSV is used by the majority of maternal-fetal medicine specialists. It is measured on a weekly basis and to be plotted in graph developed by Mari et al the until a level of 1.50 MoM is reached, at which point fetal blood sampling with possible transfusion is indicated.
Moise, Jr. Management of Rhesus Alloimmunization. Obstet Gynecol 2002.VOL. 100, NO. 3, SEPTEMBER 2002
FBS allows direct access and absolutely precise fetal values of hematocrit, direct Coombs, fetal blood type, reticulocytes count, and total bilirubin.
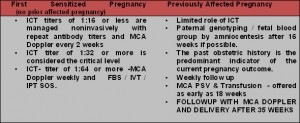
Any Rh negative mother attending the antenatal OPD will be of any of following two groups
The first Rh-D sensitized pregnancy has the best possible out than the subsequent pregnancy as they will be associated with the more and more worsening fetal anaemia due to increase in the antibody titer than previous pregnancy. So it is important for us to pick up the first affected pregnancy in antenatal clinic rather than previous affected pregnancy.
Fetal anemia is a sequel of the immunological response set in due to Alloimmunization process in the fetus leading to hemolysis. Fetus tries to compensate by increasing erythropoiesis (medullary as well as extra- medullary sites).Correction of fetal anemia is done by intrauterine transfusion. There are two methods of fetal transfusion:
In 1963, first intraperitoneal transfusion (IPT) was done by Liley. He though that Transfused RBCs will be getting in to venous circulation by sub diaphragmatic lymphatic channels.
After the invention of the ultrasound machine the management of Rh Alloimmunization has changed completely. Fetal circulation was directly accessed by putting a needle in the umbilical vein. This approach has the best possible results and so it has replaced the IPT by intravascular transfusion (IVT).
In 1984, Rodeck and colleagues introduced intravascular transfusion (IVT) facilitated by Fetoscopy. Fetoscopy guided procedures were associated with high complication rate.
Real time ultra sound guided intra venous transfusion (IVT) has made complete difference in the management.
Ultrasound guidance is used to locate the best access site.
The sites can be
In 1963, first intraperitoneal transfusion (IPT) was done by Liley. He though that Transfused RBCs will be getting in to venous circulation by sub diaphragmatic lymphatic channels.
After the invention of the ultrasound machine the management of Rh Alloimmunization has changed completely. Fetal circulation was directly accessed by putting a needle in the umbilical vein. This approach has the best possible results and so it has replaced the IPT by intravascular transfusion (IVT).
In 1984, Rodeck and colleagues introduced intravascular transfusion (IVT) facilitated by Fetoscopy. Fetoscopy guided procedures were associated with high complication rate.
Real time ultra sound guided intra venous transfusion (IVT) has made complete difference in the management.
Ultrasound guidance is used to locate the best access site.
The sites can be
After aseptic precaution A 20-guage, 7-inch long echo tip spinal needle is then guided into the targeted vessel under ultrasound guidance. After correct placement of needle fetal blood is aspirated for immediate Haematocrit, CBC, blood type and Rh factor. Usually O Negative, leukocyte depleted,Cytomegalo virus (CMV) free, HIV ,HbSAg, Hep C, tested packed Red Blood cells with hematocrit of about 80 %(to prevent over load) and irradiated ( prevent Graft-versus-host reaction)blood to preferred for the fetal transfusion.
Fetal anesthetic agent, if required, may be injected to induce fetal paralysis. Fetal anesthesia can be help to stop the fetal movement. This approach is not useful in cases where placenta is attached on anterior wall of uterus. Atracurium / vencuronium in the dose of 0.1 mg/kg is to be given.
If this is below 0.5 MoM, the tip of the needle is kept in the lumen of the umbilical cord vessel and fresh, packed blood is infused manually into the fetal circulation through a 10-ml syringe and a transfusion set. The goal is to reach a fetal Haematocrit of 40% (and up to 55% depending on the institution); the amount of blood necessary varies according to initial Haematocrit and fetal weight. To minimize potential increases in fetal cardiac over load, a transfusion Hematocrit of 80 – 90% is used, and post-transfusion fetal Haematocrit should not exceed 55%. During this time, an increase in umbilical venous pressure by 1.7 to 4.6 mm Hg has been documented to occur along with a 25% decrease in cardiac output.
Following formula can be used to calculate the volume of the blood to be transfused:
Fetoplacental volume x (Hematocrit final-Hematocrit initial)
Hematocrit in transfused blood
Fetoplacental volume = fetal weight in grams x 0.14
Software like Astraia Gmbh, GE –View point can calculate the amount of blood to be transfused once data regarding the initial fetal hematocrit is entered.
The estimated fetal weight in grams using ultrasound can be multiplied by a factor of 0.02 to determine the volume of red cells to be transfused to achieve a hematocrit increment of 10%.
At the end of the transfusion, a further fetal blood sample is aspirated to determine the final hemoglobin concentration. Knowledge of exact hematocrit after the fetal transfusion will determine the timing of the next transfusion as 1% of fetal hematocrit will be reduced due to disease process in the fetus so fetus will require the repeat transfusion. This is particularly important after 2 fetal transfusions.
2% pregnancy loss is associated with the procedure. As this is associated with 50 % FMH the anti body titer will increase after procedure.
Till desired fetal maturity is reached, usually 35 weeks, fetus is delivered and is to be shifted to NICU.
After fetal transfusion, ascites is the first to revert and last is placentomegaly.
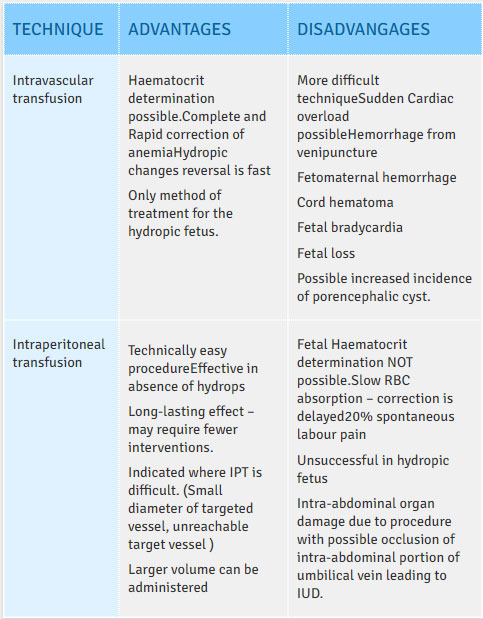
Intraperitoneal transfusion (IPT) was first performed in 1963 and IPT has significantly contributed to improved survival of fetuses affected by Rh disease. Ultrasound is used to direct a 20-gauge needle into the fetal peritoneal cavity just above the fetal bladder.
There is Compromise in ability to absorb RBCs through the lymphatic vessels in hydropic fetus. The daily absorption of red cells in the nonhydropic fetus is limited to 10% to 12%, leading to slow recovery from severe anemia.
IVT remains the procedure of choice, especially in the hydropic fetus. However, IPT should be considered in the special circumstances noted.
Follow up requirement and timing of subsequent transfusions depends on Fetal Hb reached at the completion of the procedure as 1% fetal Haematocrit (.33 gms of Hb) will be reduced due to disease process. Further developing anemia in the fetus should be monitored by MCA PSV. In most of the cases subsequent transfusions will be required every 2 to 3 weeks.
Fetal survival will depend upon
Short Term
Janssen and coworkers reported an overall survival rate of 83.7% after IVT. The influence of hydrops and severity of fetal anemia on outcome was demonstrated in perinatal mortality.
An evaluation of the relative statistical value of a set of clinical parameters in determining fetal survival in a group of 86 transfused fetuses (RhD) revealed that the best predictor of fetal loss was hydrops (OR 8.7), followed by a hemoglobin deficit of more than 6 g/dL (OR 3.2).
Mild hydropes defined by only mild Ascites had survival rate of 98 % versus severe hydropes (gross Ascites with pericardial effusion, generalized edema) had 55%. Although fetal Haematocrit was not significantly different between the two groups, the survival was higher for fetuses with mild hydrops compared with those who had severe manifestations.
Survival was positively associated with at higher number of transfusions and a younger gestational age at first transfusion.
Reversal of hydrops, which occurred in 65% of all fetuses, resulted in a survival rate of 98% irrespective of the severity of the hydropic changes.
Special mention regarding the use of uterotonics like Methly Ergometrian maleate , Oxytocin and prostaglandins :
Use of the above mentioned drugs will increase the chance of FMH ,but it is better to prevent the PPH than preventing FMH.As excessive FMH can be taken care by giving the additional prophylaxis than standard dose .
Expert and experienced specialist in the field should be involved in the management.
Fetus may suffer from
Hypo regenerative anemia due to suppressed bone marrow and erythropoiesis with accompanying low reticulocyte counts possibly due to intramedullary destruction of red blood cell precursors, bone marrow suppression from IUT, and a relative erythropoietin deficiency.
Thrombocytopenia
Jaundice
DIC
Prematurity
Fetal infection
Fetus may require phototherapy, exchange transfusion and top up transfusion with RBCs also
No complications were noted up to 62 months of follow up after IUT. There was no correlation between the global development quotient and the severity of disease, including presence of hydrops and number of transfusions. A normal neuro-developmental outcome can be expected in 95 % cases thereby justifying aggressive treatment of fetuses with severe hemolytic disease including hydrops.
